|
|
|||||||
Basic Chemistry of calcareous skeleton formation |
|||||||
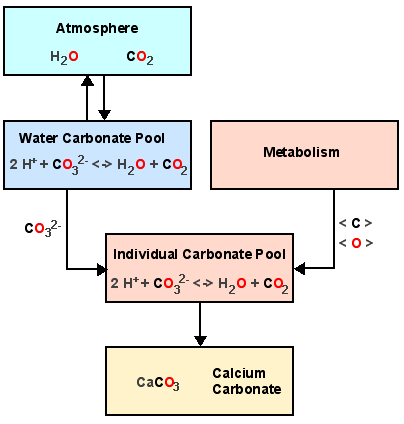
Calcareous skeletons consist of calcium carbonate CaCO3 (Calcite or Aragonite). The question is, where do the carbon C and the oxygen O come from? To understand this, we best look backwards starting with calcium carbonate CaCO3. |
|||||||
|
|||||||
The carbonate component CO32- of the shell calcium carbonate CaCO3 is taken from the individual´s carbonate pool, where we have a balance between carbonate CO32- and water H2O + carbon dioxide CO2. This individual pool is fed directly from two sources, from the carbonate pool of the surrounding water and from metabolism, which process oxygen and carbon derived from food. Note that finally all oxygen and carbon comes from the water via the autotrophs at the bottom of the food web, but isotopic composition may have been altered by metabolic processes. This effect is negleglible as long as the surrounding water is the major direct source of carbon and oxygen used for skeleton formation. This, however, depends on the species under investigation and may change e.g. with individual age (Krantz et al. 1987). As there are much more water molecules H2O that carbon dioxide molecules CO2 in the water, almost all oxygen in CaCO3 stems from H2O, whereas the carbon in CaCO3 stems from CO2. |
|||||||
|
|||||||